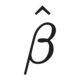Electronic Data Capture (EDC) in Clinical Trials: Everything to Know
Introduction Electronic Data Capture (EDC) systems are vital tools in modern clinical trials, designed to collect, manage, and store clinical research data electronically. This technology has largely replaced traditional paper-based…
Bioequivalence Studies and Their Role in Drug Development
Introduction Bioequivalence is a term used in pharmacology to assess the similarity of two drug formulations in terms of their bioavailability when administered to the body. Bioavailability refers to the…
Pharmacovigilance : Masters, Online Courses and Trainings
Introduction Pharmacovigilance, also known as drug safety, is a comprehensive term that encompasses the collection, analysis, monitoring, and prevention of adverse effects in drugs and therapies. It is a scientific…
Real-World Evidence (RWE) And Real-World Data (RWD)
Introduction In the pharmaceutical industry, real-world evidence (RWE) and real-world data (RWD) are distinct terms often used interchangeably to describe observational data from clinical practice. Let’s explore their differences and…
Pharmacovigilance : An In-Depth Guide
Introduction In healthcare and pharmaceuticals, ensuring the safety and efficacy of medical treatments is paramount. Pharmacovigilance, the science of monitoring and assessing the safety of drugs and medical products, plays…
Factor Levels or Binary Variables? A Case-Study in Statistical Modelling of Bipolar Disorder and the Aging Brain
When do you code your variables as levels in a factor, and when do you enter them as binary variables in a model? As it turns out, it isn’t necessarily…
Top Contract Research Organizations (CROs) or Clinical Research Organizations
A Contract Research Organization (CRO) is a firm specializing in providing clinical trial services for the pharmaceutical, biotechnology, and medical device industries. While various types of CROs exist, those catering…
Steps of Drug Development and Biostatistics
In the realm of drug development, biostatistics emerges as an indispensable tool, meticulously shaping the trajectory of potential therapies from their nascent discovery to eventual market approval. This article navigates…
Phases of Clinical Research in Drug Development
Developing new, innovative drugs is a time-consuming process, averaging around 12 years from discovery to market. Drug development unfolds through four distinct phases: discovery, preclinical studies, clinical development, and market…
Clinical Trial Monitoring
Clinical trial monitoring is a methodical and crucial process that ensures the integrity, quality, and safety of participants in clinical trials. This meticulous oversight involves reviewing trial conduct, data collection,…
Biostatistics in Clinical Research and Trials
Biostatistics stands as an indispensable element in the landscape of clinical research and trials, transcending mere post-analysis applications. Its roots trace back to the 17th century, evolving into a pivotal…
Biostatistics for Pharmacists : Books, Articles and Resources
The Vital Role of Biostatistics in Shaping Pharmaceutical Practice; Navigating the dynamic world of pharmaceuticals requires more than just a prescription pad and a wealth of medical knowledge. In the…